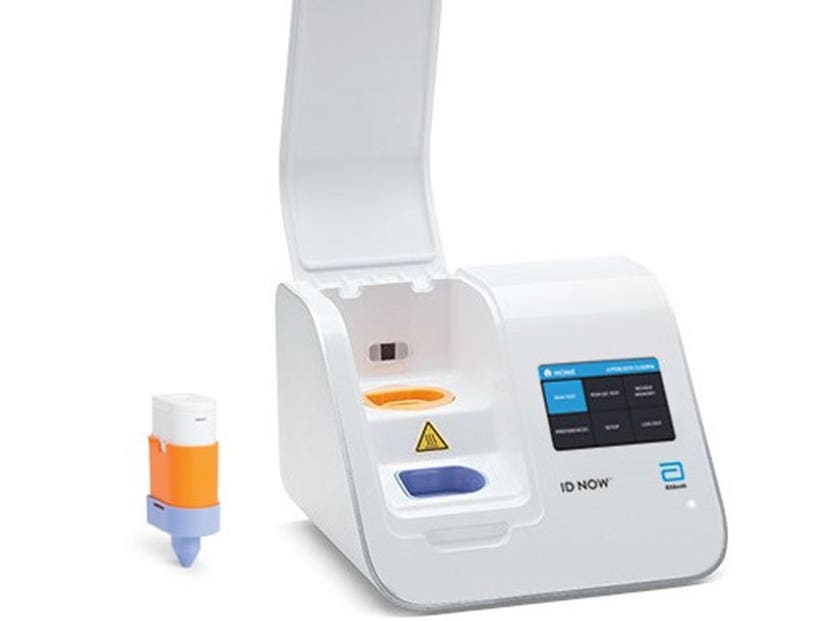
The machine is the size of a small toaster – so isn't restricted to just hospitals labs.
A test that can detect the coronavirus in five minutes arrives this week.
On Friday the FDA gave emergency authorization to Abbot Laboratories to begin mass producing what will be the fastest available molecular point-of-care test for COVID-19.
As well as its speed, the other huge advantage is the samples can be read by the lab's portable ID Now machine - which weighs 6.6lbs and is the size of a small toaster – allowing physicians' offices or urgent care clinics to conduct tests rather than just hospitals.
 YouTube
YouTube
John Krasinski, Steve Carell Have Virtual Office Reunion as John Highlights 'Good News' Amid Pandemic
View StoryThe test works by taking a swab from the nose or back of the throat, and loading it into a cartridge that fits in the palm of your hand.
"A chemical solution cracks open the virus, releasing its genetic material for ID NOW to read," a video explaining the process claims.
"Thanks to the technology behind molecular testing, if even a small amount of COVID-19 is found in the sample, ID Now works to replicate the small section of the virus' genetic material, making it detectable."
This amplification technique allows it to cut testing times from hours or even days to just minutes; the machine will tell if you are positive in five minutes, or negative in 13 minutes.
 Getty
Getty
Lady Gaga, Ellen DeGeneres and More Join Elton John's Star-Studded Living Room Concert on Fox
View StoryThe lab vowed to begin churning out 50,000 COVID-19 tests per day, starting April 1. They did not say if they were going to start producing more of the ID Now machines; there are around 18,000 units currently spread throughout the country, normally used for testing for Influenza A&B, Strep A and respiratory syncytial virus (RSV).
"Through the incredible work of teams across Abbott, we expect to deliver 50,000 COVID-19 tests per day to healthcare professionals on the front lines, where testing capabilities are needed most," said Chris Scoggins, senior vice president of Rapid Diagnostics with Abbott. "Portable molecular testing expands the country's capacity to get people answers faster."
Confirming the emergency authorization, FDA Commissioner Steve Hahn said it was a potential "game-changer" in fight against the novel coronavirus.
Got a story or a tip for us? Email TooFab editors at tips@toofab.com.